Although this appears to be very promising, there are a number of questions that I have about this study. The use of eGFR as a primary endpoint is questionable. 98% of the patients in this study were on an ACE/ARB and, as Matt alluded to in his previous post, if you took all of these patients off the ACE/ARB, you would probably see an increase in GFR. This is not necessarily a good thing if, in fact, it is simply leading to increased intraglomerular pressure. In the paper, the authors state that the drug is similar in structure to the cycloprostenone prostaglandins. Although these are described as being primarily anti-inflammatory in nature, could they also cause afferent arteriolar vasodilatation and therefore have a significant hemodynamic effect? The authors point out that the effects persisted up to 4 weeks after stopping the drug which suggests that the effect is not entirely hemodynamic but does not exclude it. It is important to note that this increase does not appear to be related to changes in creatinine metabolism. In a prior, smaller study, the same authors reported increases in measured creatinine clearance in patients treated with the drug without any change in overall creatinine excretion.
More important is the issue of protein excretion. Microalbuminuria is the often the first sign of diabetic nephropathy and our treatments are targeted at reducing albumin excretion. In this study, bardoxolone reportedly caused a ‘slight but significant’ increase in the ACR that positively correlated with the GFR. Apart from this statement, there are no primary data in the main paper. In the appendix, the table below was published (click to enlarge):
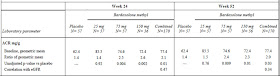
I am open to correction but I take this to mean that the ACR was 1.4 times baseline in the placebo group but that this increased to 2.6 times baseline in the 75mg group. This was highly significant. The figure below, also from the appendix, tells a similar story but it must be pointed out that the scale on the left is a log scale such that any increase may appear less than it actually was. In a study of the progression of diabetic nephropathy, I would have imagined that the change in the ACR would be an important endpoint and would have been included more prominently.
(Click to enlarge)

All that said, the result of this study matches with animal data that suggested a protective effect for this drug in models of acute and chronic renal disease and if the increase in GFR is real and beneficial, this class of medications could revolutionize the treatment of CKD. It’s great to see potential new lines of therapy for our patients and we should look forward to the phase III trials that will apparently begin enrolling later this year.

If this is true then I agree that it would be a pretty big deal for our specialty. If however the MOA of eGFR increase is something that further hastens renal damage then well...that would be a foolish and expensive mistake. The phase 3 trial as I heard from some investigators participating in the trial is an an event driven composite endpoint study. If the MOA of improved eGFR is increased intraglomerular pressure that would be pretty evident in a renal endpoint study. If the MOA is repair then perhaps it may improve outcomes.
ReplyDeleteHere is what I don't get. The company who owns it and that has been developing it, did they actually consult a nephrologist when designing the studies? It is puzzling to me that they did not think to measure GFR in their earlier studies. Seems a bit naive to think a nephrologist would accept changes in eGFR as a primary endpoint.
Great post,
ReplyDeleteAmazing work, as usual by a nephrologist. There is always tons of stuff hidden behind the abstract and the paper.
The company was smart to report only eGFR and hence get another study and get "rapid" approval from FDA. You only need to market the abstract.
Thanks for this gr8 post.
Such cynicism! The reported changes in eGFR seem too dramatic to just explain away as merely hemodynamic effect in absence of major concurrent (correlating) blood pressure changes, (and the earlier reports suggest statistical independence here.)
ReplyDeleteThe ACR findings are the most problematic, but in a field with such a dismal track record--we could perhaps do better than to smugly deride a promising development--which will at least perhaps lead to some fundamental discoveries.
As Nephrologists, we have been badly burnt by not asking the right questions before rolling out treatments (like Epo) to patients. I think your skepticism is healthy and appropriate, and the issues raised have a sound pathophysiological basis.
ReplyDeleteI would take a very different position to the last poster... in a field with such a dismal track record, we must insist on the highest quality evidence before accepting a new treatment, lest we repeat the problems of the past.
Where are the animal studies showing benefits of bardoxolone treatments on chronic renal insufficiency? If they are available no reference is made to them on PubMed.
ReplyDelete