 There are two major types of intercalated cell in the collecting duct of the nephron – the alpha and beta intercalated cells. We are probably most familiar with the alpha intercalated cells in terms of acid-base handling by the kidney. They have a luminal H+ATPase enzyme and a basolateral anion exchanger. This polarity allows them to pump H+ into the lumen, facilitating acid excretion, which is equivalent to reabsorption of bicarbonate.
There are two major types of intercalated cell in the collecting duct of the nephron – the alpha and beta intercalated cells. We are probably most familiar with the alpha intercalated cells in terms of acid-base handling by the kidney. They have a luminal H+ATPase enzyme and a basolateral anion exchanger. This polarity allows them to pump H+ into the lumen, facilitating acid excretion, which is equivalent to reabsorption of bicarbonate. Thursday, February 24, 2011
Pendrin – role in distal bicarbonate secretion
 There are two major types of intercalated cell in the collecting duct of the nephron – the alpha and beta intercalated cells. We are probably most familiar with the alpha intercalated cells in terms of acid-base handling by the kidney. They have a luminal H+ATPase enzyme and a basolateral anion exchanger. This polarity allows them to pump H+ into the lumen, facilitating acid excretion, which is equivalent to reabsorption of bicarbonate.
There are two major types of intercalated cell in the collecting duct of the nephron – the alpha and beta intercalated cells. We are probably most familiar with the alpha intercalated cells in terms of acid-base handling by the kidney. They have a luminal H+ATPase enzyme and a basolateral anion exchanger. This polarity allows them to pump H+ into the lumen, facilitating acid excretion, which is equivalent to reabsorption of bicarbonate. Wednesday, February 23, 2011
Measures of dialysis dose
 As previously discussed on RFN, the urea Kt/V is a measure of dialysis dose that is related to patient outcome. There are several different Kt/Vs encountered in the dialysis literature.
As previously discussed on RFN, the urea Kt/V is a measure of dialysis dose that is related to patient outcome. There are several different Kt/Vs encountered in the dialysis literature. spKt/V = single pool
eKt/V = equilibrated
stdKt/V = weekly standard
A nice way to think about each one is in the context of the major trials in which they were used.
spKt/V
The National Cooperative Dialysis Study (NCDS) published in 1981 examined four different 3x week dialysis prescription targets in 151 patients. The original paper did not use Kt/V. Instead, the prescription targets were high vs low time averaged BUN and long vs short dialysis treatment times.
The time averaged BUN and dialysis times achieved were approximately 90 mg/dl vs 50 mg/dl and 4.5 hours vs 3.25 hours in the high vs low and long vs short groups respectively. Protein intake was not randomized and was meant to be between 0.8 and 1.4 g/kg though some patients fell below this range.
The study showed that patients in the high BUN groups were hospitalized and withdrawn from the study protocol at statistically significant higher rates. Time was not a statistically significant variable for either of these outcomes though the p value for increased risk of hospitalization in the short time group was 0.06. The original NCDS paper did not sort out whether people in the each of the BUN groups were there because of their dialysis dose or because of their protein intake.
A subsequent reanalysis in KI by Gotch and Sargent in 1985 separated these variables out using the single pool Kt/V (for dose) and normalized protein catabolic rates (for protein intake). As seen below they showed that poor outcomes were associated with a spKt/V of less than 1.0 in 3x per week dialysis.

In practice the spKt/V is calculated for a single run of dialysis using known variables as inputs in any of several developed equations. The commonly used Daugirdas equation…
spKt/V = -ln(R - 0.008*t) + (4 - 3.5*R)(preBW-postBW/preBW)
Uses the known variables of…
R (post dialysis BUN/pre dialysis BUN)
preBW (pre dialysis body weight)
postBW (post dialysis body weight)
t (treatment time)
The spKt/V is used in most dialysis units to assess dose for patients on 3x week dialysis schedules. There are however, other versions of Kt/V that are seen in the literature and are useful in certain situations. On that note, stay tuned for eKt/V…
Monday, February 21, 2011
Cut out the salt

Sunday, February 20, 2011
Whole Lot of Pressure

The maintenance of mean arterial pressure to prevent tissue dysoxia and conserve organ function is central to the management of the critically ill. In patients with increased capillary permeability this is often achieved by administration of large volumes of IV fluids. However, resuscitation volumes of >5 litres in the first 24 hours are associated with raised intra-abdominal pressure (IAP). Although Conall & Nate have mentioned it before, a recent review of intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) by Mohmand & Goldfarb presents an excellent opportunity to review the topic.
IAH is defined as sustained or repeated elevation of intra-abdominal pressure >12mmHg, and ACS as an IAP >20mmHg associated with new organ dysfunction. IAP is easy to measure, either through the transduction of pressure in the bladder via an indwelling urinary catheter, or using an NG tube in patients whom bladder pressure measurement is not feasible.
Estimates of the prevalence of IAH and ACS suggest figures of approx. 60 and 10% respectively in non-selected ICU populations. As well as large volume fluid resuscitation, risk factors for IAH/ACS include trauma, abdominal surgery, mechanical ventilation, increased abdominal contents due to ileus or ascites, and increased capillary leak secondary to sepsis, pancreatitis, coagulopathy etc.
The review of Mohmand & Goldfarb collates a number of studies showing that IAH is an independent predictor of both mortality and the development of AKI. Indeed, they report on one study, which found that IAH was the best single predictor of the development of AKI after shock.
As regards management; severe ACS requires abdominal decompression by laparotomy, whereas medical strategies to reduce IAH include drainage of intra or extra-luminal contents, reduction of capillary leak and improvement of abdominal wall compliance. Given the link with volume expansion it is tempting to suggest a role for renal replacement therapy and ultrafiltration. However, good quality data is currently in short supply and probably all that can be surmised from existing studies is that aggressive continuous venovenous haemodiafiltration can be used to reduce IAP.
Clearly, IAP is extremely important to bear in mind when approaching AKI in the ICU. However, whether renal replacement therapy will be able to offer improvements in outcomes requires significant further study.
Wednesday, February 16, 2011
Fabry's Disease – modes of inheritance
 Just a quick piece to review the inheritance pattern of Fabry's disease, a relatively rare, but under-recognized cause of End-Stage Kidney Disease in adults.
Just a quick piece to review the inheritance pattern of Fabry's disease, a relatively rare, but under-recognized cause of End-Stage Kidney Disease in adults. Tuesday, February 15, 2011
Primer on Posterior Urethral Valves
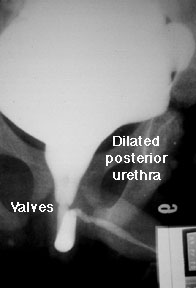 As usually happens when the renal fellows help with the medical student nephrology block, I found myself confronted last week with questions I couldn't answer. One involved the pathophysiology of posterior urethral valves (PUV), which came up in a clinical case. In case there are other fellows who, like me, tend to avoid postrenal conditions in favor of studying more glamorous parenchymal diseases, here is a short primer on PUVS.
As usually happens when the renal fellows help with the medical student nephrology block, I found myself confronted last week with questions I couldn't answer. One involved the pathophysiology of posterior urethral valves (PUV), which came up in a clinical case. In case there are other fellows who, like me, tend to avoid postrenal conditions in favor of studying more glamorous parenchymal diseases, here is a short primer on PUVS.Monday, February 14, 2011
Take one glass of water, three times a day...
 As mentioned previously by Nate, there is some evidence that lowering urine osmolarity below that of the serum can reduce the rate of growth of cysts in PCKD and thus preserve renal function. The rationale for this is that ADH stimulates cAMP production in the collecting duct and that this is required for cyst growth. Therefore, if you can reduce ADH secretion, you might be able to delay progression of the disease.
As mentioned previously by Nate, there is some evidence that lowering urine osmolarity below that of the serum can reduce the rate of growth of cysts in PCKD and thus preserve renal function. The rationale for this is that ADH stimulates cAMP production in the collecting duct and that this is required for cyst growth. Therefore, if you can reduce ADH secretion, you might be able to delay progression of the disease.Sunday, February 13, 2011
Please Take a Moment To Remember Nate Hellman
"Life is eternal and love is immortal; And death is only a horizon, And a horizon is nothing save the limit of our sight."
Saturday, February 12, 2011
Try this in clinic…
 It was the usual sort of day in clinic and the team was seeing a middle aged woman with stable diabetic nephropathy and subnephrotic proteinuria. Her blood pressure and blood sugars had been well controlled since the last visit and being good nephrologists her urine specimen was spun and the sediment examined.
It was the usual sort of day in clinic and the team was seeing a middle aged woman with stable diabetic nephropathy and subnephrotic proteinuria. Her blood pressure and blood sugars had been well controlled since the last visit and being good nephrologists her urine specimen was spun and the sediment examined.  The team was shocked to find a packed field shown top left… Which became stranger under polarized light shown to the right…
The team was shocked to find a packed field shown top left… Which became stranger under polarized light shown to the right… Some of these objects were sort of hexagonal like cystine crystals but the patient had never had a kidney stone, never had this finding before and was much older than one would expect for a cystinuria presentation. The maltese cross finding was odd as well. Cystine crystals don't have these. The objects didn’t really look like oval fat bodies and the crosses were not the clean symmetric looking ones typically seen in these fat droplets.
The team, perplexed, split up taking the slide to the urinalysis lab to ask the techs if they knew what the heck this was, hitting pubmed and back to the patient to see if there was any funny business with the specimen.
On reconvening the answer was clear: corn starch. The techs instantly said they see it all the time when their gloves contaminate a specimen. Pubmed, gave us a nice case report from NDT Plus and the patient noted having some vulvar irritation and was likely using a corn starch based baby powder which had dropped into the specimen cup.
This is the part you can try at clinic…
To confirm our discovery I dipped one of our powdered gloves in water and then prepared it like a regular urine specimen. Perfect match. Give it a try in clinic next time you have some housestaff or unsuspecting renal co-fellows around.
Wednesday, February 9, 2011
Voting is over for the 2010 Medical Weblog Awards
ACE-inhibitor induced hyponatremia
Tuesday, February 8, 2011
Urine electrolytes in metabolic alkalosis
 No time like the present for a quick review of urine electrolytes. Is there any such thing as a ‘normal’ urine sodium? Not really – like all great answers in medicine, ‘it depends’. In general, in patients who are euvolaemic, the urine sodium excretion will directly correlate with the degree of dietary sodium ingestion.
No time like the present for a quick review of urine electrolytes. Is there any such thing as a ‘normal’ urine sodium? Not really – like all great answers in medicine, ‘it depends’. In general, in patients who are euvolaemic, the urine sodium excretion will directly correlate with the degree of dietary sodium ingestion.Sunday, February 6, 2011
Alimentary Azotemia Redux: A Quantitative Approach
 The issue of whether a marked elevation in the BUN when compared with the creatinine might represent gastrointestinal bleeding was nicely covered previously on RFN. One of our attendings recently had our group of first year fellows review the issue using a quantitative approach that highlighting the relevant physiology.
The issue of whether a marked elevation in the BUN when compared with the creatinine might represent gastrointestinal bleeding was nicely covered previously on RFN. One of our attendings recently had our group of first year fellows review the issue using a quantitative approach that highlighting the relevant physiology. Consider a 72kg male in steady state eating 90grams of protein per day with a creatinine clearance of 120ml/min and a Urea clearance of 60ml/min.
Remembering that a male will produce about 20mg/kg of creatinine a day, our 72kg male will produce 1440mg of creatinine in a day…
72kg x 20mg/kg = 1440mg
A person in steady state must excrete what they produce (a key nephrology concept). So if our man makes 1440mg of creatinine he must excrete 1440mg of creatinine (if he fails to excrete it all his plasma creatinine concentration will rise and he has fallen out of steady state).
We can additionally estimate the amount of BUN produced by remembering that urea nitrogen production is approximately 1/6th of protein intake. So our man eating 90grams of protein produces 15grams of urea nitrogen each day (90grams x 1/6 = 15grams) which in steady will be excreted.
With the above we can now calculate the plasma creatinine and BUN concentrations using the clearance equation…
clearance (C) = [urine concentration (U) x urine volume (V)] / plasma concentration (P)
C = UV/P
Plug in the numbers correcting the units along the way for Cr…
120ml/min = (1440mg/day) / P
P = (1440mg/day) / 120ml/min
P = (1440mg/day) / 172,800ml/day
P = 0.0083mg/ml
P = 0.83 mg/dl
Same deal for BUN…
60ml/min = (15g/day) / P
P = (15g/day) / 60ml/min
P = (15,000mg/day) / 86,400ml/day
P = 0.17mg/ml
P = 17 mg/dl
A final thing we can sort out from what was provided is the fractional excretion of urea which by convention is expressed in percent. This is just what it says it is, the fraction of filtered urea (we'll approximate GFR with CrCl) that gets excreted in the urine. As urea is freely filtered this is…
FeUr = (Urea clearance / GFR) * 100
FeUr = (Urea clearance / CrCl) * 100
FeUr = [(60 ml/min) / (120 ml/min)] * 100
FeUr = 50%
So here’s what we know in table form…

Now imagine that our man starts feeling unwell, stops eating and has a one liter bleed from a peptic ulcer into his GI tract. For arguments sake lets say this occurs with no drop GFR (the “it’s the blood not the renal function” argument).
His protein intake is now the protein content of 1L of blood. 40% is cells (mostly rbcs) and 60% is plasma. The major proteins in the cellular and plasma parts respectively are hemoglobin and albumin (there's a bit more protein around from globulins and so on but this will give us a rough estimate).
Normal hemoglobin and albumin concentrations would be 14 g/dl and 4 g/dl respectively. So from the above we can estimate the protein content of blood in the GI tract…
1L * 0.60 = plasma volume
0.6L = plasma volume
plasma volume * protein concentration = plasma protein content
0.6L * 4g/dl = plasma protein content
0.6L * 40g/L = plasma protein content
24g = plasma protein content
1L * 0.40 = cellular volume
0.4L = cellular volume
cellular volume * protein concentration = cellular protein content
0.4L * 14g/dl = cellular protein content
0.4L * 140g/L = cellular protein content
56g = cellular protein content
Total protein content = cellular protein content + plasma protein content
Total protein content = 24g + 56g
Total protein content = 80g
Using our previous calculations our table now looks like this…

Notice that in the above scenario the BUN drops a bit as the protein intake has decreased. What if we kept our man eating the same diet and had him bleed at the same time while holding kidney function stable?

If you almost double the protein intake you almost double the BUN (from 17 to 33 mg/dl). Now let’s try the stopped eating, 1 liter bleed scenario along with a 50% drop in GFR due to hypotension. Remember that in the volume depleted state the fractional excretion on urea is typically less than 35% and for arguments sake we’ll make it 20% in our man.

As compared with no renal dysfunction we now get an BUN/Cr ratio of 23 as compared to 18. How about we run scenario with continued eating, 1 liter bleed and now with 50% drop in GFR due to hypotension with the associated drop in urea clearance.

Pretty impressive, huh? With a bit of kidney dysfunction added into increased urea production we’ve now got a BUN/Cr ratio of 98 vs 40.
The point of all this is that the BUN and serum creatinine will vary based on:
1) Cr production
2) Cr clearance
3) BUN production
4) BUN clearance
The integration of these four things yields the BUN and serum creatinine values and the subsequent ratio between the two.
As noted by Ernest, the dog paper he reviewed and the math above the most impressive BUN/Cr ratio elevations are generated by a combination of increased urea nitrogen production and decreased urea clearance. The ratio is further accentuated by the proportionally greater drop in urea clearance vs creatinine clearance seen in volume depletion.
Friday, February 4, 2011
The power of T
Wednesday, February 2, 2011
Bone pain after transplant
 Keeping the bone theme:
Keeping the bone theme: A 50-year-old woman with ESRD secondary to PKD underwent a living related-kidney transplant one-month prior. At clinic visit, she was complaining of severe throbbing pain in her hands and feet. The pain was worse on weight bearing and exertion. Physical exam was unremarkable with no joint erythema, edema or tenderness. Her creatinine was 0.8 mg/dL and metabolic parameters were remarkable for mild hypercalcemia, nl phosphate levels, mildly increased Alk Phos and PTH of 70 pg/mL. This presentation led to this brief review of potential causes of bone pain in the transplant population.
One of the most worrisome bone complications in transplant patients is avascular necrosis. Its incidence is about 5.5% and it usually presents with hip or groin pain exacerbated by weight bearing. Diagnosis requires an MRI and more than 60% of patients that develop AVN will need a joint replacement. The bilateral nature of the pain and the involvement of feet and hands make AVN an unlikely diagnosis on this case.
Kidney transplant recipients are also at increased risk of fractures. To give you an idea, the overall fracture risk after renal transplantation is 360-380% higher than in healthy individuals and is 30% higher during the first 3 years after transplantation than in patients on dialysis. Interestingly, in one survey of 600 patients, the most common site of fracture was the foot and different than postmenopausal women, bone mineral density is not a good predictor for the risk fracture. This has to do with the inability of BMD to evaluate the quality of the bone (architecture, turnover, composition and mineralization), only measuring the density of the bone. The patient’s pain was too diffuse to be related to a fracture.
What else are we missing?
Osteomalacia caused by severe vitamin D deficiency, severe hyperparathyroidism or rapid osteopenia from high-dose corticosteroid therapy could trigger some bone pain, nonetheless her vitamin D level was normal, PTH was not very high and she had only received a short course of steroids and was steroid-free at this point.
Finally, the condition of immunosuppression-related bone marrow edema syndrome came up (also known as posttransplant bone marrow edema syndrome and calcineurin inhibitor pain syndrome). This syndrome typically presents with symmetrical pain in knees or feet associated with mildly elevated alkaline phosphatase and normal ESR/CRP. The pathophysiology is not clearly understood but it seems to be related to intra-osseous vasoconstriction. CNIs have been raised as possible culprits. A MRI imaging can usually confirm the diagnosis, showing bone marrow and periarticular soft-tissue edema and absence of avascular necrosis. Despite the possibility of severe symptoms, this condition usually regresses spontaneously, being most prevalent on the first three months after transplant. Our patient was transitioned to sirolimus without improvement. After reduction of immunossuppression, her symptoms gradually improved in the following 3 months, with associated resolution of edema on MRI.
In summary, bone pain is a common complication after transplantation and in defined cases, a MRI is required for further diagnosis, specifically to exclude avascular necrosis and possibly confirm bone marrow edema if early after transplant.
Figure: MRI showing bone marrow edema.
Evaluation of primary aldosteronism: seeing is not believing
Tuesday, February 1, 2011
Dialysis membrane-induced thrombocytopenia
 As part of the regular hemodialysis prescription, we as providers have to prescribe the type of membrane we want used for the procedure. This decision is mostly based on what membranes our dialysis unit chooses to buy – though the majority are using synthetic polymer based membranes currently.
As part of the regular hemodialysis prescription, we as providers have to prescribe the type of membrane we want used for the procedure. This decision is mostly based on what membranes our dialysis unit chooses to buy – though the majority are using synthetic polymer based membranes currently.



















