A young woman with h/o polysubstance abuse and seizures was admitted to
hospital with status epilepticus. She was treated with a propofol infusion at
5mg/kg/hr which was maintained due to perceived continuing seizure activity. On hospital day 5, she developed an acute metabolic deterioration –
rhabdomyolysis : CPK 100,000, AG metabolic acidosis (HCO3 18) and non-oliguric
AKI. Her EKG also became abnormal (RBBB). The diagnosis was propofol infusion
syndrome.
What is propofol Infusion Syndrome (PRIS)?
Like many syndromes, PRIS is a conglomeration of clinical and
biochemical manifestations. Based
on 83 case reports from 1992-2007, PRIS
is characterized by:
1) Metabolic acidosis, (pH less than 7.30 or HCO3 less than 19)
2) Rhabdomyolysis (CPK more than 10K)
3) Renal failure
4) Cardiac dysfunction (Brugada-like EKG pattern, asystole, PEA,
sustained VTs, heart failure, or bradycardia)
5) Hypertriglyceridemia (TG more than 400)
6) Hyperkalemia
7) Hypotension (or use of vasopressor agent)
8) Hepatic transaminitis
9) Hypoxia (PO2 less than 60mmHg).
The first 5 manifestations are the most common. While there is no
requirement for the number of clinical manifestations a patient must exhibit to
meet diagnosis for PRIS, a
prospective study showed that most patients exhibit
at least 3 defining manifestations within 3 days of propofol use.
In the same study, the
incidence of PRIS was found to be a low 1% --similar to other estimates.
However, PRIS is associated with high
mortality, up to 30% in some
studies. Moreover,
because many of these manifestations are common, the presence of any of them
could be attributed to another etiology, thus delaying diagnosis of PRIS.
For instance, in the case vignette, the
initial rise of CK was attributed to seizure rather than PRIS.
Pathophysiology of PRIS
Inhibition of electron flow along the mitochondrial electron transport
chain/ impairs oxygen utilization. Propofol or its metabolites inhibits fatty-acid oxidation leading to buildup
of toxic fatty acid intermediates. As described in
this nice review.
Risks of PRIS?
Critical illness (especially, CNS illness); Use of propofol dosage more
than 4 mg/kg/hr —the usual adult maintenance dose is 0.3 to 3mg/kg/hr; duration
of Propofol use greater than 24hr; exogenous cathecholamines and
corticosteroids; poor intake of carbohydrate, see
this reference.
Management of PRIS
Early recognition/diagnosis; Cessation of propofol infusion; Cardiopulmonary
support; Hemodialysis (strongly advocated by expert opinion and outcome of case
series).
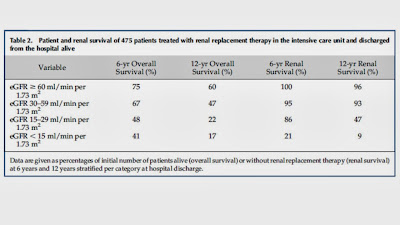
However, there are no known RCT of use of renal replacement therapy in
the treatment of PRIS.
Nonetheless, in
case series, survivors of PRIS are more likely to have received HD/CVVH.
Therefore, prolonged use of HD/CVVH is worth
considering by renal consult service.
Because propofol is lipophilic and has volume of distribution of 20-40L,
it is poorly cleared by HD/CVVH, prolonged RRT is likely needed for PRIS
management.
The patient in the case was treated with CVVH overnight, and then
transitioned to intermittent HD. At the
time of her discharge, she was off HD with her Cr back to baseline.
See
this previous post by Nate on PRIS.
Posted by Opeyemi Olabisi