Hydrochlorothiazide (HCTZ) is the most commonly prescribed anti-hypertensive worldwide and the most frequently prescribed diuretic in the United States. More than 95% of prescriptions are for doses of 25mg or less. Despite these unequivocal prescribing patterns, HCTZ has rarely been studied at these low doses, particularly as monotherapy, and has yet to be compared head-to-head with thiazide-like diuretics such as chlorthalidone or indapamide. In contrast, multiple landmark trials have demonstrated consistent reductions in cardiovascular (CV) morbidity and mortality with thiazide-like diuretics as dosed in contemporary practice.
While no trial has directly compared low-dose HCTZ monotherapy with a thiazide-like diuretic, the MRFIT trial offers the closest comparator with the results offering cautionary lessons regarding the benefits of HCTZ at historically prescribed doses (50-100 mg/day). Published in 1982, patients were randomized to placebo or combined lifestyle/drug therapy with HCTZ or chlorthalidone as the initial agent. Not only did HCTZ treated patients have higher all-cause mortality rates compared to those in the chlorthalidone arm but there was a trend towards increased all-cause mortality when HCTZ patients were compared to placebo. Moreover, when those randomized to HCTZ were switched to chlorthalidone, rates of death from coronary artery disease fell below those recorded in the placebo arm.
In contrast, nearly every seminal trial showing improved CV outcomes with diuretics has utilized the thiazide-like diuretics chlorthalidone or indapamide at contemporary daily doses of 25 and 2.5mg, respectively (ADVANCE, HDFP). The SHEP trial was the first large study to address the treatment of isolated systolic hypertension with more aggressive blood pressure goals; compared to placebo, those randomized to chlorthalidone had far fewer CV events. The ALLHAT study, which compared the efficacy of full dose chlorthalidone (25mg/d) with lisinopril (40mg/d), norvasc (10mg/d), and doxazosin (arm terminated prematurely), found that chlorthalidone resulted in the lowest blood pressure among treatment groups. It was not only as effective as the other agents in preventing the primary CV outcome but was superior with respect to the secondary outcome of heart failure (although this may have been related to improved blood pressure control rather than the agent itself). Moreover, truly elderly patients appear to tolerate the more potent thiazide-like diuretics. For example, the HYVET trial was the first study to evaluate the effects of tighter BP control among (relatively healthy) individuals over 80. Those treated with an indapamide-based regimen not only had far fewer fatal strokes but lower rates of adverse events compared to those on placebo.
Small “proof of concept” studies offer possible explanations for these differences in outcome. In a 24-hour ambulatory blood pressure monitoring study comparing equi-potent doses of HCTZ (12.5mg day) and chlorthalidone (6.25mg/day), chlorthalidone distinguished itself by its extended duration of action resulting in blood pressure control throughout the 24-hour period. The pharmacologic property is critical as agents with 24-hour efficacy protect against the morning “surge” in blood pressure, a time when patients are most vulnerable to stroke and myocardial infarction 26821625).
In light of the unequivocal therapeutic efficacy of thiazide-like diuretics and the lack of evidence supporting HCTZ as mono-therapy, the nephrology community should serve as the exception to the aforementioned trend in thiazide prescription patterns. Concern that chlorthalidone/indapamide may be too potent for the elderly are well founded but as the above trials in the aged demonstrate, low-dose therapy (e.g. indapamide 1.25mg/d) can be prescribed, allowing for less volume depletion and metabolic sequelae while providing the extended anti-hypertensive response that may be the key to their superiority over HCTZ.
Conflicts of Interest: None
Hillel Sternlicht, MD
Hypertension Specialist
Author, Concepts in Hypertension Newsletter- Subscribe for free
Showing posts with label hypertension. Show all posts
Showing posts with label hypertension. Show all posts
Saturday, April 28, 2018
Hydrochlorothiazide—Its time is up
Tuesday, April 11, 2017
Hypertension in Setting of Elevated Intracranial Pressure: to Treat or Not to Treat?
A patient has an elevated intracranial pressure (ICP) and is also markedly hypertensive. You are faced with a dilemma??
Which antihypertensive agents would be preferred in a patient with known elevated intracranial pressure?
Should we even be trying to lower blood pressure in this setting in the first place?
When tight blood pressure control is directly tied to neurological and cardiovascular outcomes, the ideal approach should be intensive-care monitoring and a continuous infusion. We are quick to make such a recommendation for hypotensive patients requiring vasopressors but often are reluctant to make similar recommendations of patients on the other end of the blood pressure spectrum.
In the setting of increased in ICP due to neurological emergencies, the agents of choice all have two things in common: short half-life and neutral effect on ICP. Continuous infusions of labetalol and nicardipine are effective antihypertensive agents which do not increase ICP in this setting and are easily titratable. Agents such as hydralazine, nitroglycerin, and sodium nitroprusside all act as cerebral vasodilators and thus increase ICP despite lowering blood pressure. Reviewing the current medication regimen, hydralazine may be a poor choice of antihypertensive agent while clonidine and carvedilol seem to have a neutral effect on ICP.
Should we even attempt to lower blood pressure in this setting? Cerebral perfusion pressure (CPP) is related to mean arterial pressure (MAP) and intracranial pressure (ICP) as follows:
*Note that ICP is often measured in cm H2O (or CSF) but in this equation must be mmHg. The conversion is 1 mmHg = 1.36 cm H2O.
Per the above equation, acute drops in MAP may result in decrease in CPP. The Cushing reflex is the constellation of hypertension (predominantly systolic with a wide pulse pressure) and bradycardia as a response to marked increase in ICP as an effort to maintain CPP (late stages of this reflex also include apnea which completes the Cushing's triad). Per UpToDate recommendation, CPP should remain greater than 60 mmHg and hypertension should only be treated when CPP is greater than 120 mmHg.
A hypothetical patient with a BP of 200/90 has a MAP of approximately 127 mmHg. If the ICP is >7 mmHg (~9.5 cm H20), the CPP is still less than 120 mmHg. One could argue not acutely lowering BP in this patient.
Furthermore, the presence of longstanding hypertension may alter the autoregulation set-point. Accustomed to mitigating elevated MAP by vasoconstriction, cerebral vasculature may not be able to accommodate a rapid decrease in MAP, thus increasing risk of ischemic injury. However, traumatic brain injury and stroke can also damage the cerebral vasculature's ability to autoregulate potentially raising ICP further in setting of uncontrolled hypertension.
In summary, management of hypertension in setting of elevated ICP is controversial and partially dependent upon the reason for elevated ICP. The choice of antihypertensive in this setting should be one that does not cause an increase in ICP and ideally should have rapid onset of action, short half-life, and no active metabolites. Before treating HTN in setting of elevated ICP, keep in mind the goal CPP of 60-120 mmHg.
Daniel Edmonston, Nephrology Fellow, Duke University
Which antihypertensive agents would be preferred in a patient with known elevated intracranial pressure?
Should we even be trying to lower blood pressure in this setting in the first place?
When tight blood pressure control is directly tied to neurological and cardiovascular outcomes, the ideal approach should be intensive-care monitoring and a continuous infusion. We are quick to make such a recommendation for hypotensive patients requiring vasopressors but often are reluctant to make similar recommendations of patients on the other end of the blood pressure spectrum.
In the setting of increased in ICP due to neurological emergencies, the agents of choice all have two things in common: short half-life and neutral effect on ICP. Continuous infusions of labetalol and nicardipine are effective antihypertensive agents which do not increase ICP in this setting and are easily titratable. Agents such as hydralazine, nitroglycerin, and sodium nitroprusside all act as cerebral vasodilators and thus increase ICP despite lowering blood pressure. Reviewing the current medication regimen, hydralazine may be a poor choice of antihypertensive agent while clonidine and carvedilol seem to have a neutral effect on ICP.
Should we even attempt to lower blood pressure in this setting? Cerebral perfusion pressure (CPP) is related to mean arterial pressure (MAP) and intracranial pressure (ICP) as follows:
CPP = MAP - ICP
*Note that ICP is often measured in cm H2O (or CSF) but in this equation must be mmHg. The conversion is 1 mmHg = 1.36 cm H2O.
Per the above equation, acute drops in MAP may result in decrease in CPP. The Cushing reflex is the constellation of hypertension (predominantly systolic with a wide pulse pressure) and bradycardia as a response to marked increase in ICP as an effort to maintain CPP (late stages of this reflex also include apnea which completes the Cushing's triad). Per UpToDate recommendation, CPP should remain greater than 60 mmHg and hypertension should only be treated when CPP is greater than 120 mmHg.
A hypothetical patient with a BP of 200/90 has a MAP of approximately 127 mmHg. If the ICP is >7 mmHg (~9.5 cm H20), the CPP is still less than 120 mmHg. One could argue not acutely lowering BP in this patient.
Furthermore, the presence of longstanding hypertension may alter the autoregulation set-point. Accustomed to mitigating elevated MAP by vasoconstriction, cerebral vasculature may not be able to accommodate a rapid decrease in MAP, thus increasing risk of ischemic injury. However, traumatic brain injury and stroke can also damage the cerebral vasculature's ability to autoregulate potentially raising ICP further in setting of uncontrolled hypertension.
In summary, management of hypertension in setting of elevated ICP is controversial and partially dependent upon the reason for elevated ICP. The choice of antihypertensive in this setting should be one that does not cause an increase in ICP and ideally should have rapid onset of action, short half-life, and no active metabolites. Before treating HTN in setting of elevated ICP, keep in mind the goal CPP of 60-120 mmHg.
Daniel Edmonston, Nephrology Fellow, Duke University
Saturday, February 25, 2017
Awesome HTN Trial Cheat Sheet and info for becoming an ASH Specialist in Clinical Hypertension
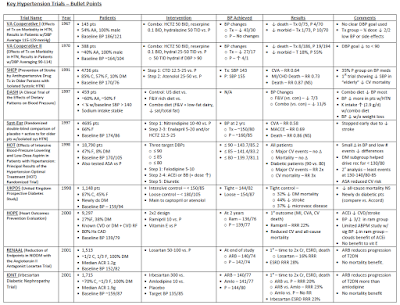
For folks (including fellows in training) who are perhaps more interested in hypertension than the average nephrologist, certification from the American Society of Hypertension (ASH) can offer a greater understanding of hypertension physiology and management (through preparation) as well as a notch in your CV and expertise to advance your career. If you are intrigued, ASH offers some education materials online as well as an explanation of the certification process.
Trainees who have passed their ABIM boards and are enrolled in relevant fellowships (e.g. renal, cards, endo) are able to take the boards. You do not have to pass your nephrology boards first apparently.
If you are interested consider discussing this with your TPD.
Since hypertension is perhaps the most overwhelming area of nephrology, at least in terms of clinical trials, here is a quick cheat sheet of key trials. Not all encompassing but a good start.
Rob Rope, MD Nephrology Fellow, Stanford
Thursday, April 14, 2016
A Fresh Approach to Understanding Clinical Hypertension
 With advances in the field of nephrology broadening our
knowledge of transplant medicine, glomerular disease, and the interventional aspects
of access, I believe fellowship training in the traditional areas of the
specialty such as hypertension have diminished.
With advances in the field of nephrology broadening our
knowledge of transplant medicine, glomerular disease, and the interventional aspects
of access, I believe fellowship training in the traditional areas of the
specialty such as hypertension have diminished.
And yet, apart antibiotics, anti-hypertensives are the
single most important therapy contributing to rising life expectancies. Because
while ESRD trials focus on endpoints such as PTH levels and the CKD literature
uses outcomes such as a doubling of serum creatinine, hypertension studies utilize
clinical endpoints such as all cause
mortality. Moreover, it’s a
fascinating disease, our understanding of which is unrivaled by any other
condition. What is the circadian rhythm of blood pressure? How does pulse
pressure affect the risk of CAD? What is the relationship between basal heart
rate and mortality? To understand hypertension is to appreciate human
physiology, to apply evidence-based medicine, and to practice cost effective care.
Moreover, despite the proliferation of national treatment guidelines,
management is more nuanced than targeting similar blood pressure levels for all
patients and prescribing the same handful of agents. The “art” of management hinges
on understanding the difference between metoprolol and betaxolol,
hydrochlorthiazide and indapamide, and losartan and azilsartan. As such, if
there is one disease to know, and know well it’s hypertension.
To improve my own knowledge of the above, I’m currently undertaking
an additional fellowship in hypertension under George Bakris at the hypertension
center here at the University of Chicago. In order to share what I have learned,
I’ve created a bi-weekly e-newsletter, “Concepts in Hypertension,” as a non-commercial medium to convey key aspects of the
disease. Each issue is concise,
summarizing one seminal paper and
underscoring one key concept. The
newsletter then ends with a “clinical perspective” that indicates how the
selected paper informs the management of patients at our hypertension center.
Below are several recent issues. If you’d like to subscribe
to this free, non-commercial publication, you can do so by visiting www.bp-specialists.com/newsletter
Hillel Sternlicht
The University of Chicago
Wednesday, November 18, 2015
What does SPRINT mean for HTN and CKD?
There has been much fanfare regarding the release of the SPRINT trial in the last several weeks. Finally, evidence that intensive blood pressure reduction is good! But, does this apply to everyone? Is there adverse risk to lowing BP this low? Also, what does this mean for our patients in the CKD clinic?
Let’s take a look at the SPRINT study and try to answer these questions.
What was SPRINT?
SPRINT was a large randomized controlled trial with over 9,000 non-diabetic patients, funded by the NIH, to study the effect of intensive blood pressure control on cardiovascular health. The trial was terminated early (after a mean follow-up of 3.3 years) due to a significantly lower rate of the primary composite outcome (MI, ACS not resulting in MI, stroke, acute decompensated HF, or death from cardiovascular causes) in the intensive-treatment compared to the standard-treatment group. For more coverage of SPRINT go to NephJC.
The basics of the enrolled population are as follows:
What about CKD?
First, SPRINT was not designed to be a CKD progression trial. However, it did include a large group of patients with CKD. In the patients with baseline CKD, intensive blood pressure control had no effect on the composite renal outcome (reduction in eGFR of 50% or more, dialysis or transplantation), nor on the development of incident albuminuria. Indeed, in patients without baseline CKD intensive blood pressure control led to higher rates of developing an eGFR < 60 ml/min/1.73 m2 (patients had to have at least a 30% drop in eGFR). The significance of this is unclear. Importantly, in the pre-specified subgroup analysis, the cardiovascular benefits of intensive blood pressure management were similar in patients with and without CKD. The caveat to all of this discussion was that the trial was not powered to answer this question.
What were the costs of aggressive treatment?
While overall serious adverse events were not statistically more common (38% vs 37%), there were more episodes of hypotension, syncope, AKI, hyponatremia, and hypokalemia in the intensive group. Interestingly, orthostatic hypotension was actually higher in the standard treatment group and there was no increase in falls with intensive therapy. Patients were seen on a monthly basis by protocol if not at goal. Those in the intensive group had “Milepost Visits” every 6 months where the addition of medication was protocolized for patients with SBP < 120 mmHg (unless compelling contraindications existed). Notably, despite these aggressive measures half the patients were still unable to reach the intensive goal.
What did we know about aggressive BP control prior to SPRINT?
Nephrology dogma, codified in guidelines, has long argued for lower BP targets in patients with proteinuric CKD. A meta-analysis from 2011 combined the three key trials on aggressive blood pressure reduction (MDRD, AASK, and REIN-2) with a total of almost 2,300 primarily non-diabetic patients. The results suggested that aggressive blood pressure control (goals ranged approximately 125-130/75-80) on the whole does not improve CKD outcomes however sub-group analyses of proteinuric patients indicate the possibility of a benefit to stricter blood pressure control in terms of CKD progression. This possible benefit was seen in patients with greater than 0.22 g/g of proteinuria in AASK and greater than 1000 mg/d in MDRD. The recent JNC-8 guidelines argued however that this same data represented moderate quality evidence that lower BP targets do not slow CKD progression. For additional discussion of the post-hoc analyses of MDRD and AASK click here (previous review by Graham Abra on RFN). Given this potential equipoise perhaps it is unfortunate that patients with higher proteinuria were excluded from SPRINT.
What do we know then going forward?
Hypertension is a strong risk factor for CKD. While the exact goal remains unclear, controlling blood pressure to < 140/90 is likely to be beneficial. Importantly, as the nuances of SPRINT are discussed – including additional outcomes such as the effect of intensive control on cognitive function in the elderly – hypertension in general is still very undertreated. Based on a recent CDC report 65% of US adults over the age of 60 have hypertension but only ½ are controlled. While this represents improvement from around 30% at the turn of the Millennium this still means that ½ of the population is not meeting the more conservative goal of 140/90. Increasing the percentage of patients controlled to 140/90 should have a profound and meaningful effect on the incidence of ESRD. SPRINT shows us however that for some of our patients with CKD, aggressive SBP control to < 120 can provide tangible, though not dramatic, improvements in cardiovascular risk.
Post by Robert Rope, Nephrology Fellow, Stanford
Let’s take a look at the SPRINT study and try to answer these questions.
What was SPRINT?
SPRINT was a large randomized controlled trial with over 9,000 non-diabetic patients, funded by the NIH, to study the effect of intensive blood pressure control on cardiovascular health. The trial was terminated early (after a mean follow-up of 3.3 years) due to a significantly lower rate of the primary composite outcome (MI, ACS not resulting in MI, stroke, acute decompensated HF, or death from cardiovascular causes) in the intensive-treatment compared to the standard-treatment group. For more coverage of SPRINT go to NephJC.
The basics of the enrolled population are as follows:
- All patients were over the age of 50 (mean 68). 36% were female.
- Patients had elevated cardiac risk based on: a 10 year Framingham risk ≥ 15%, clinical or subclinical CVD, CKD with eGFR 20-60 ml/min/1.73 m2, or being over age 75.
- 28% of patients (~2,600) had CKD with minimal proteinuria (patients with > 1 g proteinuria or >; 600 mg of albuminuria were excluded).
- 28% of patients were over age 75 at enrollment, their mean age was 80.
- 43-45% of patients were on statins and ~50% were on aspirin.
- Over 60% of patients started the trial with a SBP of > 145 mmHg.
- Chlorthalidone was encouraged as the primary thiazide-type diuretic.
- The suggested initial triad of medications was a diuretic, CCB (preferably amlodipine), and ACEi or ARB.
Intensive Treatment
|
Standard Treatment
|
Results
|
|
Mean SBP at 1 year
|
121 mmHg
|
136 mmHg
|
|
Mean # Medications
|
2.8
|
1.8
|
|
Primary Outcome (ACS, CVA, CHF, CV death)
|
1.65 %/year
5.2 % (over 3.3 years)
|
2.19 %/year
6.8 % (over 3.3 years)
|
HR 0.75 (0.64-0.89)
RR reduction 25%/year (*driven by CHF and CV death)
NNT 61 (over 3.3 years)
|
All-Cause Mortality
|
1.03 %/year
3.3 % (over 3.3 years)
|
1.40 %/year
4.5 % (over 3.3 years)
|
HR 0.78 (0.67-0.90)
RR reduction 26%/year
NNT 90 (over 3.3 years) |
What about CKD?
First, SPRINT was not designed to be a CKD progression trial. However, it did include a large group of patients with CKD. In the patients with baseline CKD, intensive blood pressure control had no effect on the composite renal outcome (reduction in eGFR of 50% or more, dialysis or transplantation), nor on the development of incident albuminuria. Indeed, in patients without baseline CKD intensive blood pressure control led to higher rates of developing an eGFR < 60 ml/min/1.73 m2 (patients had to have at least a 30% drop in eGFR). The significance of this is unclear. Importantly, in the pre-specified subgroup analysis, the cardiovascular benefits of intensive blood pressure management were similar in patients with and without CKD. The caveat to all of this discussion was that the trial was not powered to answer this question.
What were the costs of aggressive treatment?
While overall serious adverse events were not statistically more common (38% vs 37%), there were more episodes of hypotension, syncope, AKI, hyponatremia, and hypokalemia in the intensive group. Interestingly, orthostatic hypotension was actually higher in the standard treatment group and there was no increase in falls with intensive therapy. Patients were seen on a monthly basis by protocol if not at goal. Those in the intensive group had “Milepost Visits” every 6 months where the addition of medication was protocolized for patients with SBP < 120 mmHg (unless compelling contraindications existed). Notably, despite these aggressive measures half the patients were still unable to reach the intensive goal.
What did we know about aggressive BP control prior to SPRINT?
Nephrology dogma, codified in guidelines, has long argued for lower BP targets in patients with proteinuric CKD. A meta-analysis from 2011 combined the three key trials on aggressive blood pressure reduction (MDRD, AASK, and REIN-2) with a total of almost 2,300 primarily non-diabetic patients. The results suggested that aggressive blood pressure control (goals ranged approximately 125-130/75-80) on the whole does not improve CKD outcomes however sub-group analyses of proteinuric patients indicate the possibility of a benefit to stricter blood pressure control in terms of CKD progression. This possible benefit was seen in patients with greater than 0.22 g/g of proteinuria in AASK and greater than 1000 mg/d in MDRD. The recent JNC-8 guidelines argued however that this same data represented moderate quality evidence that lower BP targets do not slow CKD progression. For additional discussion of the post-hoc analyses of MDRD and AASK click here (previous review by Graham Abra on RFN). Given this potential equipoise perhaps it is unfortunate that patients with higher proteinuria were excluded from SPRINT.
What do we know then going forward?
Hypertension is a strong risk factor for CKD. While the exact goal remains unclear, controlling blood pressure to < 140/90 is likely to be beneficial. Importantly, as the nuances of SPRINT are discussed – including additional outcomes such as the effect of intensive control on cognitive function in the elderly – hypertension in general is still very undertreated. Based on a recent CDC report 65% of US adults over the age of 60 have hypertension but only ½ are controlled. While this represents improvement from around 30% at the turn of the Millennium this still means that ½ of the population is not meeting the more conservative goal of 140/90. Increasing the percentage of patients controlled to 140/90 should have a profound and meaningful effect on the incidence of ESRD. SPRINT shows us however that for some of our patients with CKD, aggressive SBP control to < 120 can provide tangible, though not dramatic, improvements in cardiovascular risk.
Post by Robert Rope, Nephrology Fellow, Stanford
Hypertensive emergency during dialysis
Intradialytic hypertension is defined as an increase in blood pressure after dialysis initiation. It is a common condition affecting up to 15% of patients on hemodialysis. For a thorough review, look at this prior blog.
The pathophysiology is likely multifactorial:
- volume overload
- renin-angiotensin-aldosterone over activation
- endothelial dysfunction (rises in the vasoconstrictor endothelin-1)
- sympathetic over activation
- EPO-related
- net sodium gain during dialysis (hyponatremic patients dialyzed with high sodium bath)
- electrolyte disturbances (hypokalemia and hypercalcemia)
- dialytic removal of antihypertensives: agents that are significantly removed during dialysis include atenolol, metoprolol, lisinopril and enalapril. ARB, labetolol, carvedilol, ramipril, hydralazine, benazepril, clonidine and hydralazine and calcium-channel blockers have little clearance during dialysis (figure above)
Management include:
- increase time of dialysis with more gentle ultrafiltration
- attempt to lower dry weight
- review antihypertensives to favor those not eliminiated on dialysis
- consider adding ACEI/ARB
- if hyponatremia, lower sodium of dialysate
The patient above had her dialysis time increased to 4.5 hours per session, dialysate sodium was reduced to 135 (predialysis Na was 127) and an ACEI was added to her regimen. This yielded improvement in symptoms over the next few weeks and her dry weight was further lowered about 3 kg.
@LVRiella
www.leoriella.com
Labels:
dialysis,
hypertension,
Leonardo Riella
Wednesday, February 25, 2015
Blood pressure target in diabetes mellitus
Hypertension in diabetic patients increases
the risk of microvascular and macrovascular complications. It is quantitatively
and qualitatively different from the non-diabetic population and characterized
by disturbed circadian rhythm of blood pressure (BP) with increased
variability. It also features frequent nocturnal hypertension with high 24 hour
BP load and impaired auto-regulation of blood flow leading to microvascular
injury.
A large
meta-analysis of 1 million individuals followed for 14 years showed a
continuous decrease in cardiovascular risk with reduction in BP to as low as
115/75 mmHg. In the absence of RCT data, presuming “lower is better”, BP
targets of < 130/80 mm Hg were traditionally recommended in diabetic
patients. However the hypothesis of a J-shaped relationship with risk
challenges the lower BP targets suggesting that benefits of extreme BP
reductions are smaller than moderate reductions. This seems logical as
physiologically there is a low (as well as high) BP threshold for organ blood
flow auto-regulation. Two diabetic statin trials (TNT
and PROVE
IT-TIMI) reported a J-shaped relationship between BP and adverse
cardiovascular events, although there were no BP lowering interventions. Recently, JNC 8 (based on the ACCORD trial,
where the SBP target of < 120 mm Hg could have produced J shaped curve) and
ESH/ESC 2013 (diastolic target based on HOT trial) recommended a
relaxed BP target of < 140/90 mmHg in diabetic patients. These conflicting
recommendations on hypertension targets, from various professional bodies have
created confusion in the minds of physicians.
Comparison of BP targets (in mm Hg) by different guidelines |
|||
Age |
Diabetes |
Chronic Kidney disease |
|
JNC 8 (2013) |
<60 y: <140/90
|
<140/90 |
<140/90 |
ESH/ESC (2013) |
Elderly <
80y:
|
<140/85 |
<140/90 |
ASH/ISH (2014) |
< 80 y: <140/90
|
<140/90 |
<140/90 |
AHA/ACC/CDC (2013) |
<140/90
|
<140/90
|
<140/90
|
KDIGO BP guidelines in CKD (2012) |
No recommendation for general population. For Elderly
with CKD ND
|
|
|
Albuminuria
< 30 mg /24 hr
|
|||
A recent
meta-analysis in JAMA has reignited the debate of BP targets in patients
with diabetes. Emdin et al analyzed
45 RCT`s (100,354 participants), conducted between Jan 1966 and October 2014,
of BP lowering treatment in patients with diabetes (regardless of presence or
absence of defined hypertension). Trials with predominantly type 1 diabetes
patients were excluded. The researchers examined the associations between
BP-lowering treatment and vascular disease in type 2 diabetes. They found that:
- Each 10-mmHg lower systolic BP was associated with a lower risk of mortality, cardiovascular disease events, coronary heart disease events, stroke, albuminuria and retinopathy.
- All outcomes, including mortality, were reduced when SBP was lowered from elevated baseline of >140 mm Hg and higher to a range of 130-140 mmHg.
- Further reduction of SBP below 130 mm Hg yielded lower risk of stroke, retinopathy and progression of albuminuria.
- Irrespective of drug class, the associations between BP-lowering treatments and outcomes were not significantly different except for stroke and heart failure.
The authors recommended that for patients
at high risk of stroke, retinopathy or progression of albuminuria, BP treatment
should be commenced at initial SBP level of 140 mmHg and target SBP below 130
mmHg.
The lower risk of stroke with reduction of
SBP below 130 mmHg has been previously reported in the TNT
trial, this meta-analysis
and a subgroup analyses
from the ONTARGET trial. However, the bigger question is if such lower SBP
target can be achieved without any adverse events in the elderly diabetic
population. The rate of serious adverse events reported in ACCORD trial in
intensive treatment group (achieved BP 119 mmHg) was 2.5 times that of the
control group (achieved BP 133 mmHg). While there is clear benefit in BP
sensitive outcomes like stroke, it is unclear why lower SBP target below 130
mmHg does not benefit other outcomes like heart failure and renal failure. This
could be due to the fact that hypertension trials have a short follow up and
these outcomes occur too late in the disease process to see early benefits. Or
could this be due to J-shaped relationship?
As summed up in a recent commentary titled
“Hypertension
Guidelines in need of Guidance”: We should be more worried about
hypertension, not hypotension. Surely, one would avoid excessive or unwanted
degree of BP lowering in patients with hypertension; it needs only common
sense, not guideline committees.
Which hypertension guidelines do you
follow? And what BP target do you set for your diabetes patients? Will you try
to target these lower SBP if your patient tolerated them? Leave your comments
below.
Amit Langote
Nephrology Fellow, Ottawa
Thursday, March 20, 2014
NephMadness 2014 Part 1- Hypertension Bracket
So it is that time of the year again, NephMadness 2014! I am hoping it doesn’t distract me too much from the basketball…
This year’s bracket has thrown up some very interesting match ups. I had a really hard time deciding who goes through in each round. I hope our RFN readers will allow me to indulge myself and do what everyone is told NOT to do in medical school and residency, namely, review a review [article]!
Other big news this year was the long awaited JNC8 report. This had been in the pipeline for years but when it arrived it was not well received by everyone. Some members of the committee even went so far as to publish their concerns about the recommendations. A minority of the panel had concerns about raising the SBP target in over 60s (without DM or CKD) from 140 to 150mmHg.
Please leave a comment telling us about your highlights from this years NephMadness!
Saturday, January 11, 2014
Not so symple: End of the road for renal denervation procedures?
Since the advent of minimally invasive renal denervation
procedures about a decade ago, the nephrology community had been eagerly awaiting conclusion of the SYMPLICITY 3 trial. As many of you might already
know, Medtronic, the device’s company issued a press
release on January 9th about the procedure failing to meet the
primary efficacy endpoint. To a lot of us, this result has been surprising, and
disappointing to a certain extent.
 |
| 1952 paper from Mayo: Renal denervation/sympathectomy is not a new concept and has been around for almost a century. |
To understand the context, let’s review the background of
renal denervation’s role in treatment of resistant hypertension briefly. Per
NHANES data from the period 2003-2008, the prevalence of resistant hypertension
is 8.9 ± 0.6% of
the US hypertensive population, and continues to increase. I am guessing the prevalence could
theoretically be a little lower if the new, more relaxed,
hypertension treatment goals of JNC 8 are considered. This was discussed by
Matt earlier.
Renal denervation is supposed to work on
the premise that the sympathetic nervous system is hyperstimulated in patients
with treatment resistant high blood pressure, and the kidneys modulate that to a large extent. Afferent
signaling from the kidneys increases central sympathetic drive, while efferent
signals to the kidneys increase renin release and sodium retention, while reducing
renal blood flow. Hence, if you could cut off this two way traffic between the
kidneys and the sympathetic nervous system, you should be able to bring the
blood pressure down. Here is a picture that
explains this. And below is a video of how the procedure is performed:
Renal sympathetic
afferent and efferent fibers run circumferentialy in the wall of the renal artery,
and ablation reduces both pathways
We have seen a succession of studies done to validate this hypothesis. Earlier, Matt had
talked about the SYMPLICITY-1
trial, which was a non-randomized pilot study. Then we had SYMPLICITY-2
trial in 2010 which was a larger randomized study with 106 patients. This was
conducted in Europe, Australia, and New Zealand. This showed significant
reduction in office based blood pressures at 6 months, with no serious side
effects. And so, with much fanfare and hype, the SYMPLICITY-3
trial was initiated in August 2011. While the study has not been published, the authors chose
to communicate the procedure’s lack of efficacy (in meeting the primary end
point) via a press release. There was no mention about the status of secondary
end points (which included ambulatory BP changes) in the statement.
WHICH BRINGS US TO
THE QUESTION…
Why did the trial
fail to meet the primary efficacy end point, in spite of the prior data that looked promising? It
appears to be the best designed trial till date to study this issue.
SYMPLICITY-3 was bigger (535 patients) and better designed (control patients
underwent a sham procedure) than its predecessors. I wonder if that itself
could have been the reason for us seeing the lack of any benefit. That in fact,
the prior trials were outliers given smaller size of the studies, or lack of randomization. Or, could it have something to do with anatomical and functional
regrowth of sympathetic nerves that can happen after they are ablated (as can happen post transplant)? Let us know your thoughts about what you
think could have been the reasons!
IMPLICATIONS FOR CKD
PATIENTS
Something that I have always wondered about (and which has
nothing to do with this press release) is the safety and efficacy of this
procedure in CKD patients. It is plausible that if you are tinkering with the
renin angiotensin system, you could adversely affect renal function. Just like
you could be leery of giving ACE inhibitors to an advanced CKD-4 patient with
hypertension, would you consider not doing this procedure as well? Could we see
hyperkalemia develop? In the absence of adequate data, I would say that I don’t
know, but we did see a
study that tried to answer this question. However, it was
a small study (15 patients, and even those didn’t complete follow up), and a
short follow up period.
IF ALL YOU HAVE IS A
HAMMER, EVERYTHING LOOKS LIKE A NAIL?
So is this development the beginning of the end of renal
denervation procedure? Maybe; or maybe not.
Medtronic has already suspended further enrollment in other renal denervation
trials in the US (SYMPLICITY-4),
Japan, and India. However, the device will still remain available for “discretionary
use” by physicians (good luck convincing insurance companies to pay for a
procedure that is about as good as sham!). But, we still have other
potential indications under the sun which could be a breath of life for the
device/procedure. These include heart failure, metabolic syndrome, and obstructive
sleep apnea.
Finally, we also have another device that works on the principle of baroreceptor
activation that is being
actively studied for treatment of resistant hypertension.
Wednesday, December 11, 2013
UMOD, Translational Nephrology! Contender for Top 10 of 2013
Genome-wide association studies (GWAS) are large population based collaborative studies seeking genetic patterns that would explain common complex phenotypes. These large studies have given us much insight into the genetic risk profile of patients with diseases such as chronic kidney disease and hypertension. Frequently the genetic markers described as risk genotypes are single nucleotide polymorphisms (SNPs) that lie in regions of the genome yet to be ascribed a functional role. Trudu et al in Nature Medicine this month describe a beautiful set of experiments that explain how risk variants for CKD and hypertension found by GWAS effect blood pressure regulation at the molecular level.
A number of GWAS have described risk SNPs in the promoter region of UMOD (1, 2, 3,4, 5, 6, 7). The UMOD gene codes for uromodulin or Tamm-Horsfall protein, which is secreted into the urine by cells of the thick ascending loop of Henle (TAL). Uromodulin has been shown to reduce UTIs and regulate NKCC2 and ROMK, the two main channels responsible for NaCl transport in the TAL. Furthermore, UMOD mutations cause dominantly inherited CKD (MCKD2). Susceptibility variants found in the UMOD gene are at high frequency in the general population and confer a 20% increased risk of CKD and 15% risk of hypertension.
This paper set out to uncover the biological mechanism that would explain the increased CKD and hypertension in patients with these risk genotypes. They looked specifically at the 2 lead variants located in the UMOD promotor region. Briefly, in human nephrectomy samples (removed due to RCC), those with the risk genotypes had higher uromodulin expression than those with non-risk genotypes. They confirmed this association of UMOD promotor risk variants and higher urinary uromodulin in large population-based cohort (SKIPOGH). In mouse-models, mice over-expressing UMOD had higher blood pressures and more LVH than controls and had more interstitial pathology (despite normal renal function) than controls. They then showed that mice over-expressing UMOD had more active NKCC2 (furosemide sensitive channels) than controls. They also showed that the higher BP in UMOD over-expressing mice could be dropped to baseline levels by furosemide (all mice had comparable levels of ENaC and NCC). More work was done to further elucidate the mechanism of NKCC2 phosphorylation by UMOD. Finally the authors bring their attention back to humans. They used a never-treated human hypertensive cohort (MI_HPT) and stratified them according to one of the risk variant genotypes (rs4293393). Patients homozygous for the risk genotype had statistically significant higher baseline diastolic BP. Some of these patients underwent furosemide testing. Patients with the homozygous risk allele had a higher natriuretic response and diastolic BP drop than others (both statistically significant). To summarize the authors conclusions; patients with risk alleles in the UMOD promotor had greater risk of hypertension and CKD. These patients made more uromodulin. Uromodulin increases NKCC2 activity and thus salt sensitive hypertension. Also, UMOD over-expression increases interstitial kidney damage and thus increases the risk of CKD. These risk genotypes for disease are at high frequency in all ethnicities tested. The authors suggest that selective pressure for disease related variants in UMOD is similar to the APOL1 story. The protective effect of uromodulin on UTIs and ability to raise blood pressure may have lead to its selective over-expression.
This paper demonstrates the mutual importance of large-scale studies such as GWAS and studies of rare monogenetic diseases such as Bartter and Gitelman syndromes. This paper is a worthy contender of a top ten listing this year with a truly translational set of studies combining modern basic science with clinical studies.
I also want to give a shout out for stories on new targets for ADPKD and the new AHA Cholesterol guidelines.
Wednesday, August 14, 2013
Chloride: Queen of the Electrolytes
 In June's edition of JASN Jacques et al. highlighted the emerging importance of the
role of chloride in the pathogenesis of hypertension.
Their group developed a mouse model that over expressed the protein pendrin in
the aldosterone-sensitive region of the distal tubule. These mice developed
hypertension that was attributed to increased NaCl absorption driven by over
expression and increased activity of the pendrin chloride exchanger.
In June's edition of JASN Jacques et al. highlighted the emerging importance of the
role of chloride in the pathogenesis of hypertension.
Their group developed a mouse model that over expressed the protein pendrin in
the aldosterone-sensitive region of the distal tubule. These mice developed
hypertension that was attributed to increased NaCl absorption driven by over
expression and increased activity of the pendrin chloride exchanger.
Pendrin was first described as a chloride channel in the
kidney in the early 2000s. Pendrin is a chloride-bicarbonate exchange protein that
facilitates the electroneutral movement of chloride to the intracellular space
and bicarbonate to the extracellular space or urinary space. This channel is
also found in the thyroid and inner ear and is the gene that causes Pendreds syndrom.
It is now widely accepted that the pressor effects of salt
(NaCl) are dependent on Na as the major determinant of intravascular volume and
thus hypertension. It has also been demonstrated that for Na to mediate a
hypertensive effect, it needs to be in the form of NaCl (Berghoff and Geraci,
Intern Med J 56:395-397). In their study, Berghoff and Geraci showed that subjects
on a high NaCl diet but not on a high NaBicarbonate diet developed hypertension.
These experiments have been reproduced in human and animal models. Interestingly,
hypertensive and normotensive subjects switched from a NaCl diet to an
equimolar NaBicarbonate diet experienced a decrease in blood pressure.
Pendrin is normally found in the type B Intercalated cells
of the aldosterone region of the nephron. Recently published studies by the
same group suggest that pendrin can also work in tandem with the Na-dependent chloride/bicarbonate
exchanger (this is a different channel to pendrin and is also found in the CCD)
resulting in electroneutral NaCl absorption and that this process is thiazide
sensitive.
In JASNs June edition, the Jacques group showed that pendrin mediates
chloride absorption distally and that this is the driving force for Na
absorption distally either through the ENaC and/or Ndcbe channels. The
significance of their findings are that 1) chloride is required for NaCl
absorption in ‘salt sensitive’ hypertension and that 2) pendrin is the channel
that facilitates the absorption of chloride.
On the basis of this paper and other papers showing similar
findings with regard to Pendrin's role in NaCl balance the authors suggest their
work solidifies the concept of chloride-sensitive hypertension.
It must be remembered that these studies don’t dispute that
Na is primary in maintaining blood volume and driving hypertension. However,
chloride absorption is a necessary requirement for the absorption of Na in the
setting of a salt load causing hypertension. Thus, Chloride might be the queen
and Na the king of extracellular solutes!
Posted by Andrew Malone
Labels:
Andrew Malone,
electrolytes,
hypertension,
Pendrin
Subscribe to:
Posts (Atom)




















