A 60 year-old woman with a longstanding history of hypertension, scleroderma and MGUS presented to the emergency room with diarrhea, vomiting and AKI (creatinine increased from baseline of 1.5mg/dl to 3.1mg/dl). She had a distant history of membranous nephropathy diagnosed on a renal biopsy 25 years previously. She had been worked up in the renal clinic for CKD and had a renal US showing relatively small kidneys. Her medications included an ACE inhibitor.
On admission, she had no hematuria. Her BP was elevated although she had a significant postural drop. She had dipstick proteinuria and she was empirically started on oral steroids for a possible GN. A renal biopsy was performed:
The image above is of the renal cortex. A single glomerulus is seen (A) which is hypoperfused. There is significant dilatation of the tubules (B) indicating acute tubular injury. There is attenuation and degeneration of the tubular epithelial cell layer. At the lower end of the image is an atrophic tubule with a hyaline cast.
This image again shows a hypoperfused glomerulus. However, the main finding here is an extremely damaged arteriole (A). There is multilayering within wall of the vessel (that was replicated throughout the biopsy). The vascular lumen is almost occluded with endocapillary proliferation and remodeling of the vessel wall.
In some areas of the biopsy (better perfused), the glomeruli looked relatively normal. There was some mesangial expansion with irregular capillary loops but no evidence of membranous disease.
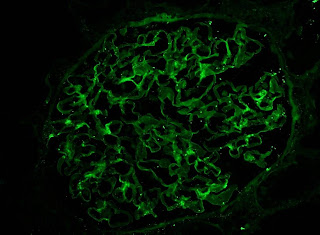
IF showed some minimal deposition of IgG in the mesangium but equal kappa and lamda light chains.
Finally, the EM showed multiple mesangial deposits (A) of uncertain significance but again, no evidence of membranous disease. Notably, there were was no evidence of active inflammation in the biopsy.
Following the report of the biopsy, the steroids were stopped. The patient's blood pressure was controlled and her renal function returned to baseline within a few days. It is likely that this acute episode was related to volume depletion and acute tubular injury exacerbated by her severe underlying vascular disease. Interestingly, she now has no albuminuria and she is back on her ACEi. The significance of the mesangial deposits remains unclear and she will be followed on an ongoing basis in the renal clinic.
We often see patients like this on consult who present with AKI following a GI illness while on an ACEi. However, we don't normally get to see the pathology in these very common case.
Click on any image to enlarge
On admission, she had no hematuria. Her BP was elevated although she had a significant postural drop. She had dipstick proteinuria and she was empirically started on oral steroids for a possible GN. A renal biopsy was performed:
The image above is of the renal cortex. A single glomerulus is seen (A) which is hypoperfused. There is significant dilatation of the tubules (B) indicating acute tubular injury. There is attenuation and degeneration of the tubular epithelial cell layer. At the lower end of the image is an atrophic tubule with a hyaline cast.
This image again shows a hypoperfused glomerulus. However, the main finding here is an extremely damaged arteriole (A). There is multilayering within wall of the vessel (that was replicated throughout the biopsy). The vascular lumen is almost occluded with endocapillary proliferation and remodeling of the vessel wall.
In some areas of the biopsy (better perfused), the glomeruli looked relatively normal. There was some mesangial expansion with irregular capillary loops but no evidence of membranous disease.
IF showed some minimal deposition of IgG in the mesangium but equal kappa and lamda light chains.
Finally, the EM showed multiple mesangial deposits (A) of uncertain significance but again, no evidence of membranous disease. Notably, there were was no evidence of active inflammation in the biopsy.
Following the report of the biopsy, the steroids were stopped. The patient's blood pressure was controlled and her renal function returned to baseline within a few days. It is likely that this acute episode was related to volume depletion and acute tubular injury exacerbated by her severe underlying vascular disease. Interestingly, she now has no albuminuria and she is back on her ACEi. The significance of the mesangial deposits remains unclear and she will be followed on an ongoing basis in the renal clinic.
We often see patients like this on consult who present with AKI following a GI illness while on an ACEi. However, we don't normally get to see the pathology in these very common case.
Click on any image to enlarge