A man in his 50s with a history of diabetes, renal stones, gastric bypass surgery and CKD stage III/IV presented to the clinic with fevers, chills and vague abdominal pain. A urine culture was positive for E Coli and he was treated with a quinolone with resolution of his symptoms. In view of his previous history of renal calculi, a CT abdomen was ordered.
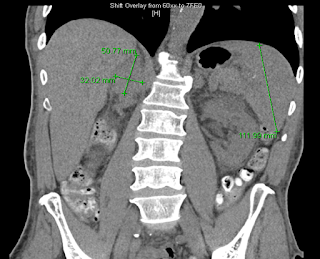
The CT scan revealed a 5 x 2.2 cm mass lesion in the right renal/suprarenal region inseparable from the kidney and the right adrenal gland and the differential diagnosis was either a RCC or adrenal carcinoma. He then proceeded to an MRI abdomen.
This again showed a mass in the right suprarenal region, inseparable from the right kidney and adrenal gland with some central necrosis. At this point, the decision was made to proceed with a partial nephrectomy for likely carcinoma.
This is a low-power view of the renal cortex. There is diffuse global glomerulosclerosis involving approximately 80% of glomeruli. There is also significant tubular atrophy and interstitial fibrosis with associated areas of inflammation.
There was focal perirenal and intrarenal scarring with disruption of the renal capsule.
The adrenal gland was essentially normal apart from some focal inflammation and adhesion to the capsule. There was no evidence of any malignancy and it is likely that the changes seen were a result of infection which had resolved by the time of the surgery.
Higher power view of the preserved glomeruli revealed changes characteristic of diabetic nephropathy - nodular glomerulosclerosis.
Interestingly, he also had many tubules containing oxalate crystals, likely related to his previous gastric bypass surgery. There was associated acute tubular injury.
This case lead to an interesting debate in our conference. Should he have been treated for a longer period with antibiotics and then rescanned prior to the resection. In the end, the consensus was that the treatment he received was appropriate. Multiple imaging studies were done that were suggestive of malignancy and he had constitutional symptoms including weight loss and fever. A review of all tumor nephrectomies performed at BWH a few years ago revealed that 1/110 cases was not actually tumor. It is hard to argue in that setting that delaying the resection is the appropriate management. Of course, in this case, there was the added complication of advanced CKD and he has now lost some of his residual GFR (although his creatinine has returned to the pre-surgery baseline).
One final image: on the MRI scan, he was incidentally found to have multiple gallstones - I just thought that the picture looked really cool.
Click on any image to enlarge